What is the compressibility factor (Z) for 0.02 mole of a van der
By A Mystery Man Writer


SOLVED: If PR=3, TR=2.0, the compressibility factor (Z) is equal to* 2 Points) a. Z=0.85 b. Z=0.55 c. Z=1.5 d. Z=0.95
At a high pressure, the compressibility factor (Z) of a real gas is usual..

Gaseous State (Real Gas) Exercise, PDF, Gases

Chemical Process Engineering - Harry Silla - Ventech!
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

Compressibility factor of the CO2 – SO2 system. Experimental results

0.585%NaCl solution at 27∘C has osmotic pressure of

Sheet - 01 - Real Gas, PDF, Gases
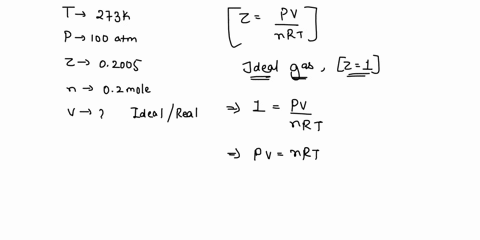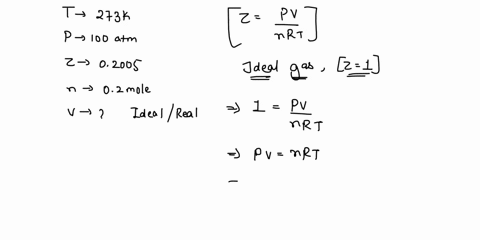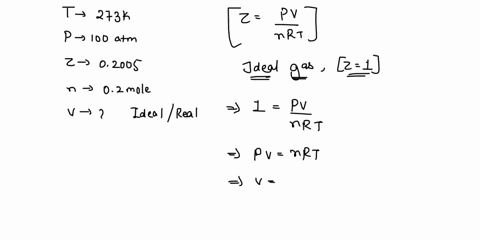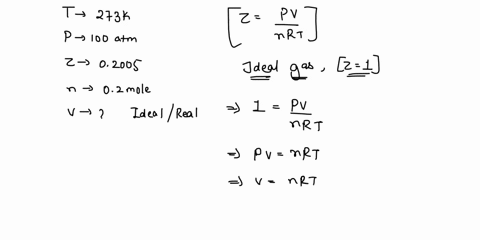
SOLVED: 17. Calculate the compressibility factor for CO2, if one mole of it occupies 0.4 liter at 300 K and 40 atm. Comment on the result. (A) 0.40, CO2 is more compressible

Van Der Waals Equation - an overview
Benzene, C6H6